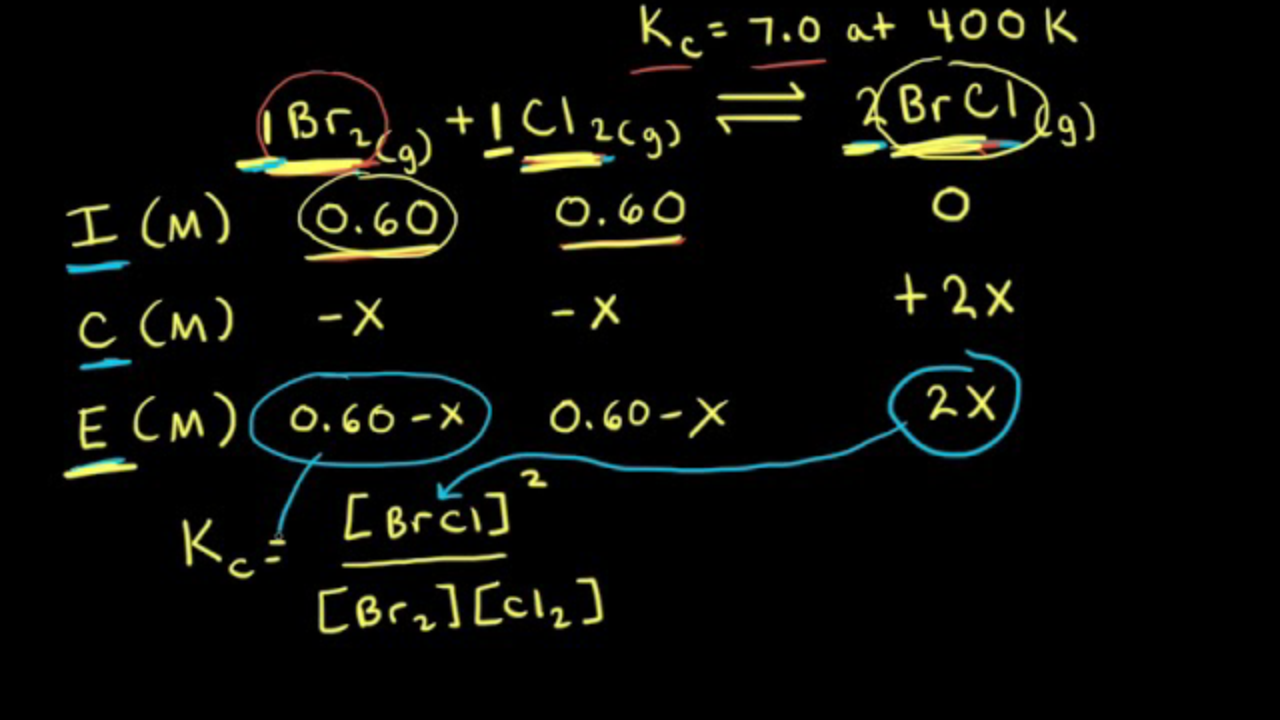
Calculating equilibrium concentrations from initial concentrations and the equilibrium constant (worked example) (video) | Khan Academy

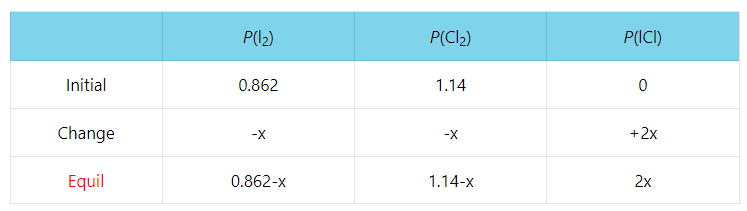
SOLVED: Setting up an ICE table to calculate Kc: Iodine molecules react reversibly with iodide ions to produce triiodide ions. I2 (aq) + 2I- (aq) ⇌ I3- (aq) If a solution with
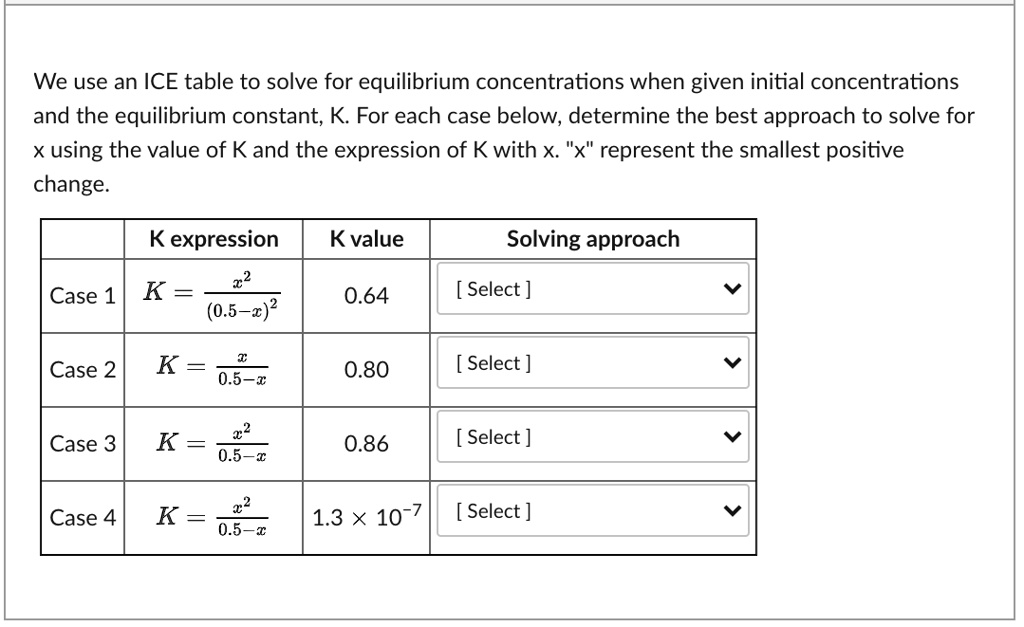
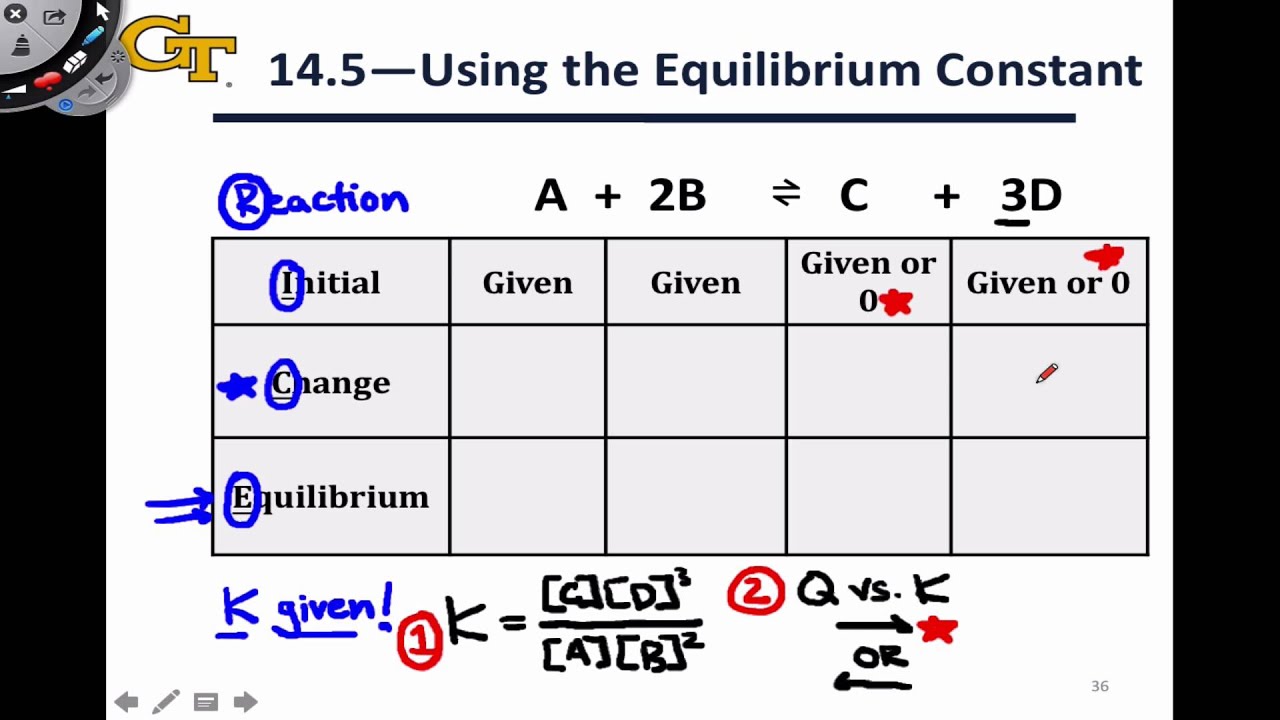
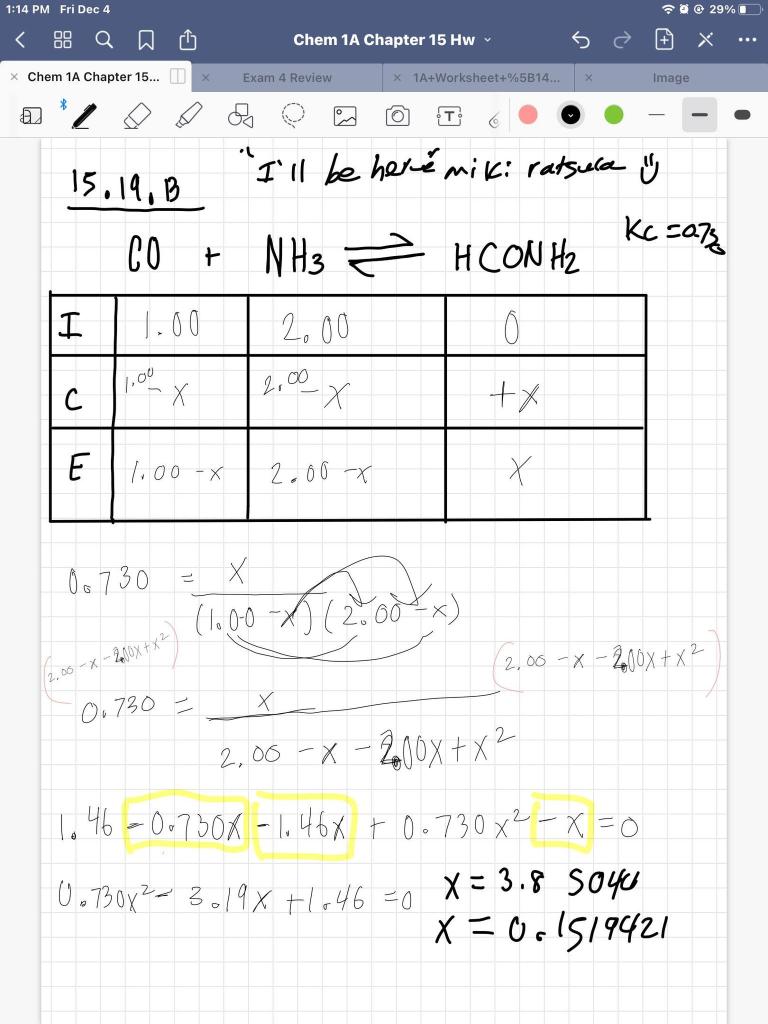
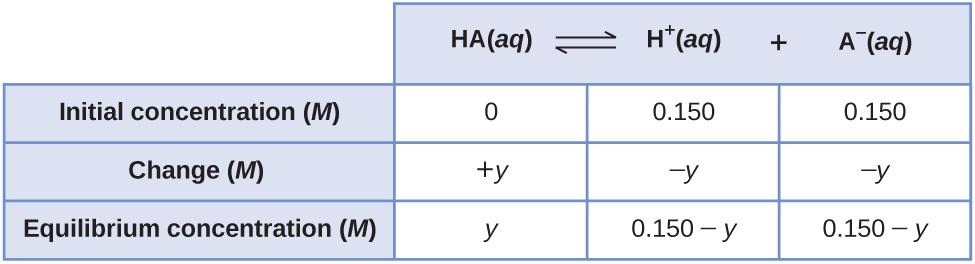
SOLVED: We use an ICE table to solve for equilibrium concentrations when given initial concentrations and the equilibrium constant; K For each case below, determine the best approach to solve for using
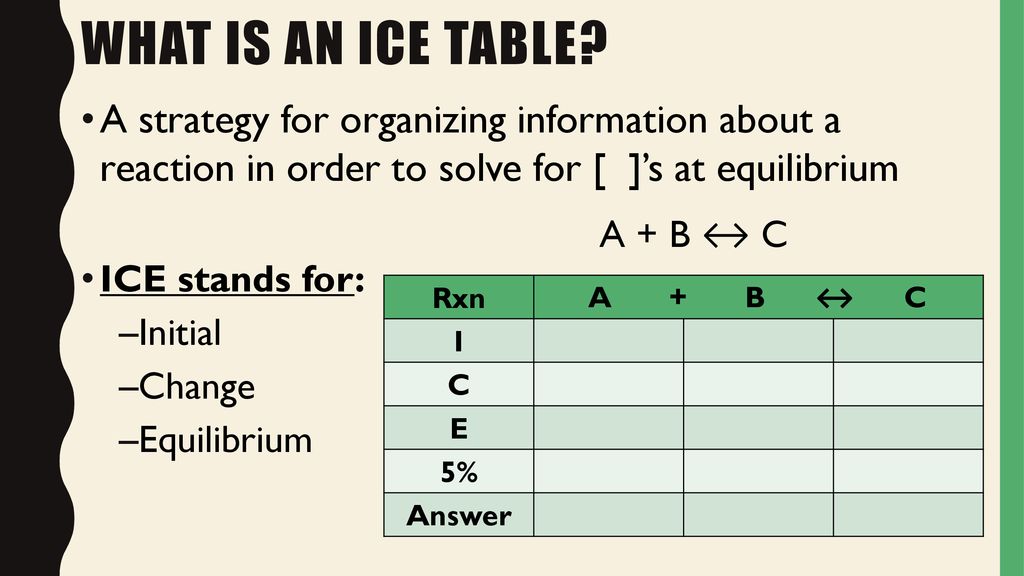
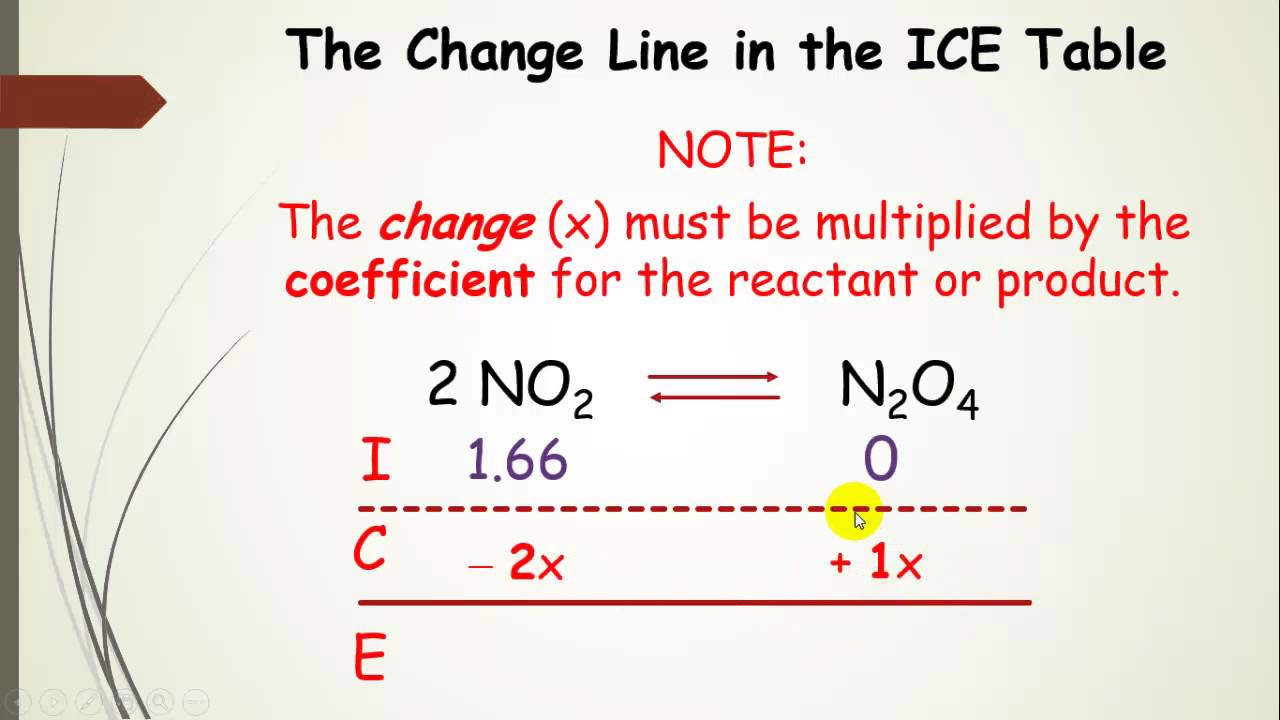
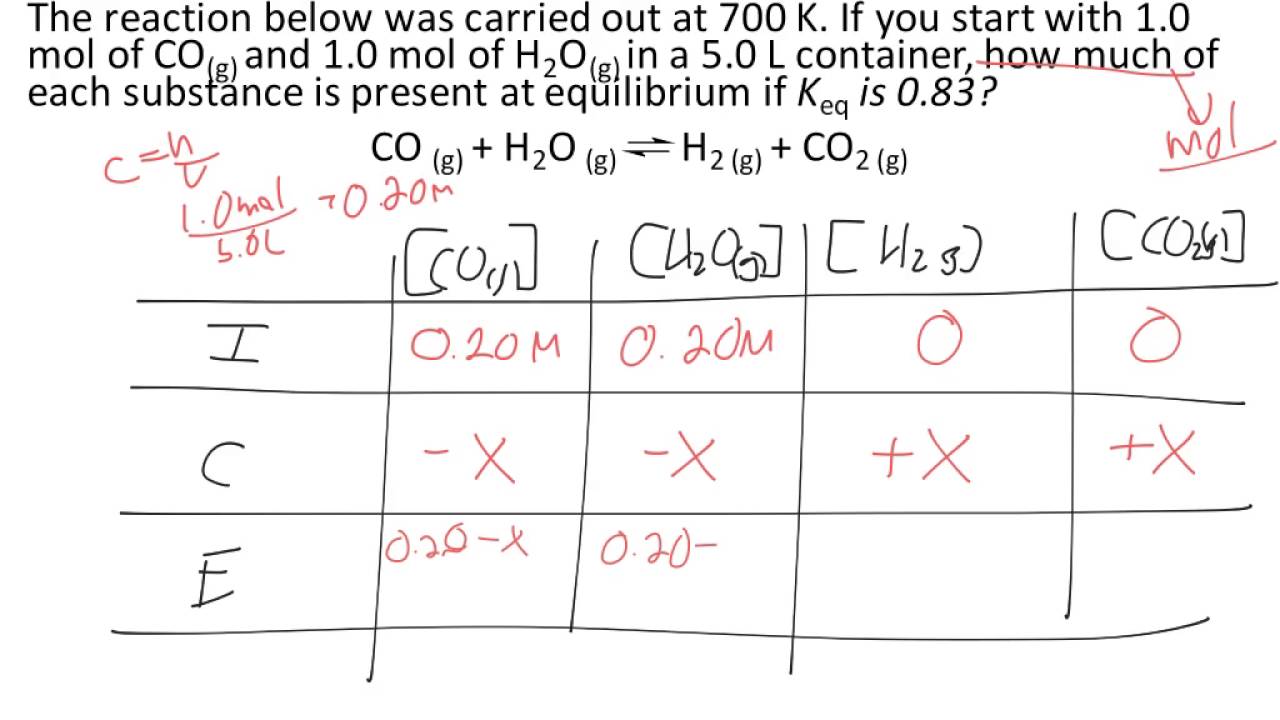
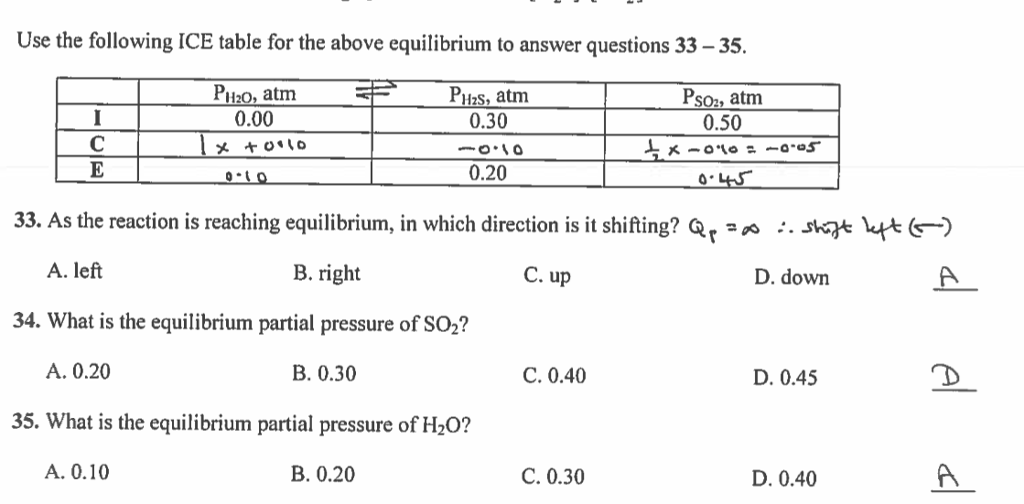
N45 ICE Tables Target: I can find the concentration of reactants and products at equilibrium using an ICE table. - ppt download
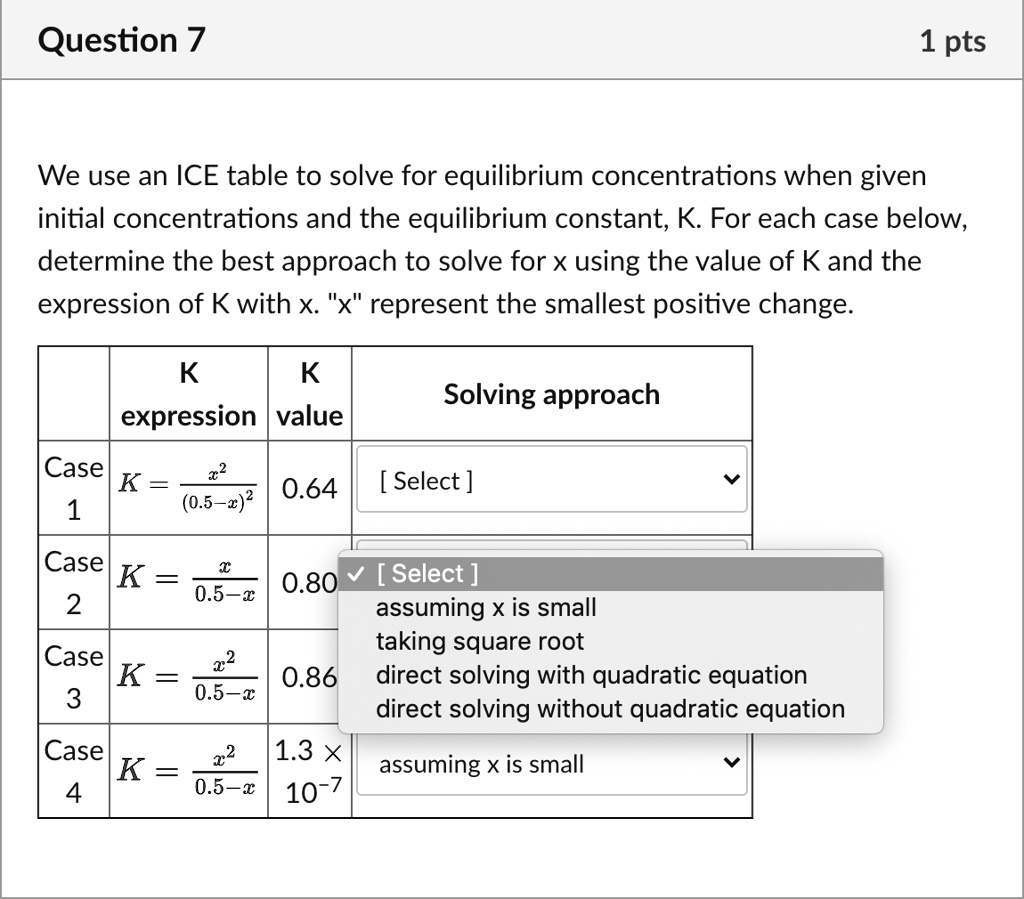
SOLVED: We use an ICE table to solve for equilibrium concentrations when given initial concentrations and the equilibrium constant, K. For each case below, determine the best approach to solve for X